Leaf polymorphism of beeches from the Jura (France) in the late Pliocene (Piacenzian): contribution to taxonomy in palaeobotany Polymorphisme foliaire des hêtres du Jura (France) au Pliocène terminal (Plaisancien) : contribution à la taxonomie en paléobotanique
Océane CLUZEAU ,
Zoé FLOCHON ,
Laura PAJOT ,
Philippe FERRANTE ,
Maxime HOFFMAN
et David DELMAIL
Fossilized beech leaves were extracted from nodules in the Bletterans plateau (Jura, France) dating back to the Late Pliocene (Piacenzian, -2.7 Ma). During this period, seasonality was much less pronounced than it is today. A warm climate allowed the development of deciduous tree vegetation, comprising a mixture of tropical, subtropical, and temperate forests without modern analogues, including the presence of Fagus and Quercus. Various qualitative and quantitative morphological traits were studied, as well as comparisons with leaves from fossil species such as F. menzelii and F. kraeuselii,and leaves from present-day species like F. grandifolia and F. sylvatica. The most discriminative features include the basal angle, the angle between primary vein and secondary veins, the angle between primary vein and tertiary veins, and the density of tertiary veins. Three distinct groups stand out, referred to as morphotypes indicating environmental variations rather than genetic groups. Leaves of F. sylvatica separate into two distinct morphotypes, whereas the American species F. grandifolia, and the European species F. kraeuselii and F. menzelii, form a third morphotype. The Bletterans samples are close to these three morphotypes. These morphological correspondences do not allow associating a species to the jurassian samples, but they support the existence of leaf morphotypes acclimated to fluctuating environmental conditions.
Des feuilles fossiles de hêtre ont été extraites de nodules sur le plateau de Bletterans (Jura, France), datant du Pliocène terminal (Plaisancien, -2,7 Ma). A cette période, la saisonnalité était beaucoup moins prononcée qu'aujourd'hui. Un climat chaud a permis le développement d'une végétation d'arbres caduques comprenant un mélange de forêts tropicales, subtropicales et tempérées sans analogues modernes, incluant la présence de Fagus et Quercus. Divers traits morphologiques qualitatifs et quantitatifs ont été étudiés, ainsi que des comparaisons avec des feuilles d'espèces fossiles telles que F. menzelii et F. kraeuselii, ainsi que des espèces actuelles comme F. grandifolia et F. sylvatica. Les caractéristiques les plus discriminantes incluent l'angle à la base, l'angle entre la nervure principale et les nervures secondaires, l'angle entre la nervure principale et les nervures tertiaires, et la densité des nervures tertiaires. Trois groupes distincts se démarquent, désignés comme des morphotypes indiquant des variations environnementales plutôt que des groupes génétiques. Les feuilles de F. sylvatica se séparent en deux morphotypes distincts, tandis que l'espèce américaine F. grandifolia, ainsi que les espèces européennes F. kraeuselii et F. menzelii, forment un troisième morphotype. Les échantillons de Bletterans sont proches de ces trois morphotypes. Ces correspondances morphologiques ne permettent pas de rapprocher une espèce des échantillons jurassiens, mais elle supporte l'existence de morphotypes foliaires acclimatés à des conditions environnementales fluctuantes.
Océane CLUZEAU, Zoé FLOCHON, Laura PAJOT: these authors contributed equally to this work
David DELMAIL: corresponding author david.delmail@univ-rennes.fr
Introduction
The genus Fagus (Linnaeus, 1753) is a small taxon comprising 10 species. They are distributed across two subgenera, Fagus and Engleriana (Denk et al., 2005), and are found exclusively in the Northern Hemisphere. Some have very broad distributions, such as F. sylvatica in Europe (Jiang et al., 2022) and F. grandifolia in North America, while others are restricted, like F. hayatae, primarily found in Taiwan with a few populations in Eastern China (Denk, 2004).
Palaeobotanical data suggest an origin of Fagus in East Asia, from where it subsequently spread to Europe, Japan, and North America (Peters, 1997). These data indicate that the genus Fagus appeared more recently in Europe compared to North America. In the early Eocene, there were ecological continuities of deciduous boreal forests between Alaska and Siberia (Tiffney, 1985). A second land bridge may explain the north-south gradient of deciduous species present on each continent, which was possibly present at the end of the Paleocene or the beginning of the Eocene, between Europe and Southeastern North America (Graham, 1964).
Traditionally, current Fagus species are morphologically defined by leaf shape, cupules, and fruits (Denk et al., 2005). However, identifying fossil leaves is a challenging task due to the morphological variability of leaves. Depending on the environment, leaves of the same species can have different shapes (Baker-Brosh and Peet, 1997), leading to intraspecific variability in addition to interspecific morphology. This variability makes the taxonomy of Fagus species highly debated, and many authors have proposed revisions within the genus (Denk, 2004).
In this study, leaf samples of Fagus from nodules in the Bletterans plateau (Jura, -2.7 Ma) will be examined. The study does not aim to identify or revise the taxonomy of the genus Fagus, but rather to biometrically analyze these specimens. To achieve this, the specimens are compared to other fossil and present-day beech species. The study aims to identify leaf morphotypes to explain intraspecific variability of the morphological traits analyzed in the collected specimens. As such, certain leaf morphological traits studied could be used as proxies for palaeoenvironmental reconstructions.
Materials and methods
2.1. Study objects (Figure 1 and Table 1)
The analysis focuses on fragments of fossilized leaves from the genus Fagus. Out of all the fossil plants found in the nodules derived from gravels, 15 fossil beech leaves were used to construct a character matrix. Some highly fragmented leaves were not used due to lack of identifiable features. It should be noted that within the rock samples, pine needles, seeds, and fragments of herbaceous leaves were also found in these nodules.
Figure 1: Fossil beech leaf from Bletterans (France). Scale bar 5 cm.
Table 1: Origin of specimens and identification codes used in multivariate analyses.
|
Species |
Original code |
Source |
Region |
Period |
Climate |
Latitude |
Altitude a.s.l. |
Diminutive |
|
F. grandifolia |
- |
Denk, 2004, Fig.4A |
N. Am. |
Current |
Cold temperate, sub-continental |
20-42°N |
40-1200 m |
FG1 |
|
F. grandifolia |
- |
Denk, 2004, Fig 4B |
FG2 |
|||||
|
F. grandifolia |
- |
Denk, 2004, Fig.4C |
FG3 |
|||||
|
F. grandifolia |
- |
Denk, 2004, Fig.4D |
FG4 |
|||||
|
F. grandifolia |
- |
Denk, 2004, Fig.4E |
FG5 |
|||||
|
F. grandifolia |
- |
Denk, 2004, Fig.4F |
FG6 |
|||||
|
F. grandifolia |
- |
Denk, 2004, Fig.4G |
FG7 |
|||||
|
F. grandifolia |
- |
Denk, 2004, Figs.4H-I |
FG8 |
|||||
|
F. grandifolia |
- |
Denk, 2004, Fig.4K |
FG9 |
|||||
|
F. kraeuselii |
U. 12522 |
Van der Burgh, 2001 |
DE |
Zanclean |
Subtropical, oceanic influence |
51°N |
NA |
FK1 |
|
F. kraeuselii |
U. 10328 |
Van der Burgh, 2001 |
FK2 |
|||||
|
F. kraeuselii |
U. 10140 |
Van der Burgh, 2001 |
FK3 |
|||||
|
F. kraeuselii |
- |
Idem, Pl.8 |
FK4 |
|||||
|
F. kraeuselii |
- |
Idem, Pl.9 |
FK5 |
|||||
|
F. kraeuselii |
SF.B 11907 |
Kvaček et al., 2020 |
DE |
Pliocene |
Cold temperate |
50°N |
NA |
FK6 |
|
F. kraeuselii |
SF.B 11963 |
Kvaček et al., 2020 |
FK7 |
|||||
|
F. kraeuselii |
SS 32 |
Kvaček et al., 2008 |
DE |
Pliocene |
Humid temperate |
49°N |
NA |
FK8 |
|
F. kraeuselii |
SS 60 |
Kvaček et al., 2008 |
FK9 |
|||||
|
F. kraeuselii |
SS 245 |
Kvaček et al., 2008 |
FK10 |
|||||
|
F. kraeuselii |
SS 233 |
Kvaček et al., 2008 |
FK11 |
|||||
|
F. kraeuselii |
SS 351 |
Kvaček et al., 2008 |
FK12 |
|||||
|
F. kraeuselii |
SS 232 |
Kvaček et al., 2008 |
FK13 |
|||||
|
F. menzelii |
U. 19168 |
Van der Burgh, 2001 |
DE |
Zanclean |
Subtropical, oceanic influence |
51°N |
NA |
FM1 |
|
F. menzelii |
U. 12854 |
Van der Burgh, 2001 |
FM2 |
|||||
|
F. menzelii |
U. 15684 |
Van der Burgh, 2001 |
FM3 |
|||||
|
F. sylvatica |
HS.1 |
Campus Beaulieu |
FR |
2023 |
Cold temperate, oceanic |
48°N |
40 m |
FS1 |
|
F. sylvatica |
HS.2 |
FS2 |
||||||
|
F. sylvatica |
HS.3 |
FS3 |
||||||
|
F. sylvatica |
HS.4 |
FS4 |
||||||
|
F. sylvatica |
HS.5 |
FS5 |
||||||
|
F. sylvatica |
HS.6 |
MNHN-P-P04026713 |
FR |
1917 |
Cold temperate, oceanic |
48°N |
125 m |
FS6 |
|
F. sylvatica |
HS.7 |
FS7 |
||||||
|
F. sylvatica |
HS.8 |
FS8 |
||||||
|
F. sylvatica |
HS.9 |
FS9 |
||||||
|
F. sylvatica |
HS.10 |
FS10 |
||||||
|
Q. lancifolia |
- |
MNHN-P-P00754121 |
MX |
1865 |
Warm temperate |
23°N |
NA |
Q1 |
|
Q. lancifolia |
- |
Q2 |
||||||
|
Q. lancifolia |
- |
Q3 |
||||||
|
Q. lancifolia |
- |
Q4 |
||||||
|
Q. lancifolia |
- |
Q5 |
||||||
|
Samples |
- |
Bletterans |
FR |
Piacenzian |
Subtropical, sub-continental with mountainous influence |
40°N |
180 m |
Hn |
2.2. Geological context
The fossils studied originate from nodules extracted in the Bletterans plateau (Jura, France), at approximately 210 meters asl, in 2021. These nodules come from a formation of coarse gravels, predominantly siliceous, with a sandy matrix typical of fluvio-glacial deposits. Locally, there are clayey levels with a patina on the pebbles, giving them an ochre/brown color due to post-depositional oxidation and strong weathering (Vinet et al., 2010).
The Pliocene-Villafranchian alluvial deposits, referring to the transition Pliocene-Pleistocene, were formed by fluvial transports during deglaciation after the alpine glacial period of Prétiglien (Vinet et al., 2010), at the end of the Piacenzian, at the base of the Donau-Günz interglacial Tiglian around -2.7 million years ago (Clair, 1976).
Within the gravel formation, numerous fossils can be found, including Molluscs such as Mesodontopsis chaixi, Frechenia (Clairiella) ducrosti, micromammals like Mimosys polonicus, Desmana aff. kormosi, and also macromammals such as Mastodon borsoni and Mastodon arvernensis (Clair, 1976).
The present course of the Seille River traces back to the Pliocene Seille valley. This valley was formed by the uplift of the Serre Massif to the east, characterized by an extensional system and the presence of normal faults (Bénévent, 1932). This indicates that the studied fossils lived near a watercourse in a valley.
2.3. Palaeoclimatic context
The flora in which the Fagus specimens are found belonged to a holarctic type. This flora was common across regions on three continents: North America, East Asia (China, Korea, and Japan), and the temperate regions of Europe. These regions had a climate during the Pliocene that was notably similar (Geissert, 1962). Furthermore, palynological studies indicate that the floras in these regions became less diverse during the Plio-Pleistocene (Bout, 1968; Geissert, 1962). Fossil mammals, particularly herbivores, have provided a precise relative chronology of floristic discontinuities during the Pliocene (Bout, 1963). The lower Piacenzian, characterized by a warm and humid climate, marked the presence of numerous forests and meadows with hypsodont herbivores, before a cooling period distinguished by more herbaceous flora and fauna turnover (Demarcq et al., 1983). The fossils studied are dated to the end of the Piacenzian during a warming period, in an environment similar to that of the Mid-Piacenzian Warm Period (MPWP).
During the MPWP, the climate was generally warmer by about 1 to 5°C, particularly in Europe and northern Mediterranean regions compared to today (Haywood et al., 2000a, 2000b). Sea levels were approximately 25 meters higher than present-day levels (Dwyer and Chandler, 2008). Precipitation anomalies were also higher than current levels (380 to 1000 mm/year) (Haywood et al., 2000a; Jost et al., 2009), although this parameter can vary significantly across space and may not have had a major influence on the locality. Seasonality was much less pronounced than today (Haywood et al., 2000a). The warm climate facilitated the development of deciduous trees, including a mixture of tropical, subtropical, and temperate forests without modern analogues, with species such as Fagus and Quercus present (Salzmann et al., 2011). Temperatures decreased over the course of the Pliocene, leading to deciduous vegetation predominance.
It is estimated that during this time, the altitude was approximately 180 meters and the latitude was 40°N (Khondkarian et al., 2004).
2.4. Other study materials (Table 1)
For biometric analysis, leaves of various Fagus species (both current and fossil) from literature sources were considered. Specifically, photographs of fossilized leaves of F. kraeuselii (13 specimens) and F. menzelii (Van der Burgh, 2001) (3 samples) were included. Additionally, leaves of the current species F. grandifolia (Denk, 2004) (9 samples) were sourced from literature.
Leaves of F. sylvatica from two sources were utilized: a branch with 5 leaves collected in 2023 on the Beaulieu campus of the University of Rennes (France, 40 m altitude, 48°N), and 5 leaves from a branch extracted from the Fournié herbarium of MNHN dating back to 1917 (MNHN-P-P04026713, Fontainebleau forest, France, 125 m altitude, latitude 48°N).
Based on morpho-anatomical criteria, the external group chosen was Quercus lancifolia (Schltdl. & Cham., 1830). This species, native to Mexico and Guatemala (Central America), shares some similar characteristics with the collected samples (presence of teeth, marginal attachment of petiole) but also exhibits distinct features. Five leaves of Q. lancifolia from the Hahn herbarium of MNHN dated 1865 (MNHN-P-P00754121, Mexico) were considered for comparison.
2.5. Acquisition of data
The fossil and current specimens were photographed (Canon EOS 6D Mark II, 70mm ima/20 ⅕ sec ISO 400 EXP 0), after marking with ammonium chloride for the fossils.
2.6. Characters studied and analyses
To create a character matrix, 19 qualitative and quantitative morphological traits were studied on the leaves as described in the Manual of Leaf Architecture (Ash et al., 1999): leaf shape, length/width ratio, petiole length, basal angle, base shape, offset at leaf base > 0.1 cm, margin type, apex of teeth, primary vein (category, diameter), secondary veins (angle, category, diameter, spacing, distribution), tertiary veins (angle, category, density, length). Quantitative variables were measured in triplicate, when applicable.
Visilog 6.4 and Mesurim 2 software were used to perform measurements of quantitative characters.
Locations (Western and Eastern Europe, North and Central America) and periods (Zanclean, Late Pliocene, and current) were considered in the character matrix, depending on the specimens studied.
The biometric study was conducted using R 2.10 software to perform a principal component analysis (PCA). This multivariate statistical method involves analyzing a dataset where observations are described by several possibly correlated quantitative dependent variables, and transforming them into new independent variables. PCA allows us to assess associations/correlations between the characters of species from the literature and the samples. Additionally, a hierarchical cluster analysis (HCA) using the Ward method, was performed to highlight taxonomic distances between the different specimens studied.
Results
The principal component axes (Figure 2) explain 84.7% of the observed variance. The first principal component (PC1) is mainly influenced by the basal angle (53.7%), angles of secondary veins (25.3%), and the density of tertiary veins (7.3%). The variables contributing most to PC2 are the angles of secondary veins (37.5%), basal leaf angle (20.2%), angles of tertiary veins (15.4%), and density of tertiary veins (15.2%). The intraspecific variation of F. grandifolia, F. kraeuselii, and F. menzelii is primarily influenced by PC1, with these three groups almost overlapping in the PCA. The 10 specimens of F. sylvatica also vary according to PC1 and are divided into two groups based on their collection year. The samples from Bletterans are oriented differently on the PCA, being mainly influenced by PC2.
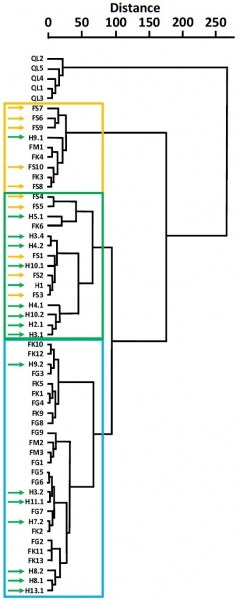
The dendrogram distinguishes three groups (Figure 3). Similar to the PCA, the F. sylvatica specimens are divided into two separate units, one containing all the samples collected in 2023 and the other those gathered in 1917. A majority of the F. grandifolia, F. kraeuselii, and F. menzelii samples (77% of the samples) are grouped together. There are a few exceptions with one specimen of F. kraeuselii (FK6) in the group of F. sylvatica collected in 2023, while specimens FM1, FK3, and FK4 are in the group of F. sylvatica collected in 1917. The most recent samples of F. sylvatica appear morphologically closer to the first group containing F. grandifolia, F. kraeuselii, and F. menzelii than to the group containing the older F. sylvatica. The Bletterans samples are present in all three groups. Among the 17 samples analyzed, 9 are similar to the F. sylvatica leaves collected in 2023, 7 are close to the specimens of F. grandifolia, F. kraeuselii, and F. menzelii, and 1 is present in the F. sylvatica group from 1917.
Discussion
4.1. Intraspecific variations
Recent studies on phylogeny (Denk et al., 2002) and morphology (Denk, 2003) of Fagus species, conclude an evolutionary radiation in the late Cenozoic era. Indeed, molecular and morphological polymorphism is observed among these species during this period (Denk, 2004). These variations are linked to several biotic and abiotic factors. Intraspecific variability can be observed across various scales. The arrival of the Fagus genus in Europe, Japan, and North America enabled specimens to colonize new ecological niches, and consequently, it is currently found across the entire Northern Hemisphere.
Leaf characteristics of woody plants can inform about the climatic conditions under which they develop (Baker-Brosh and Peet, 1997). Although genetic influence may be observed in some cases, abiotic factors such as climate, altitude, and latitude explain this intraspecific morphological variability within F. sylvatica in Europe (Peters, 1997). Leaves of F. sylvatica exhibit acclimative traits determined by climate and locality. Furthermore, species with extensive geographical ranges are likely to display greater intraspecific variation in physiology, morphology, and other parameters such as phenology and growth rate (Gratani, 2014). Gradients in the expression of certain parameters are thus observed with temperature. The distance between secondary veins observed on F. sylvatica leaves, as a measure of vein density, differs according to climate. High vein density is a consequence of climatic factors increasing water stress (Uhl, 2014). Moreover, year-to-year climate variation or differences between shaded and sunlit leaves, are factors that can explain these morphological differences.
Altitude also strongly impacts the foliar polymorphism of Fagus (Adamidis et al., 2021). The relationship between the leaf shape of European beeches and the latitude of their origin sites, suggests intraspecific variations resulting from long-term selection by local environmental conditions (Stojnić et al., 2022). Leaves from higher latitude origins tend to have shorter and wider blades, whereas specimens from southern origins tend to have longer and narrower blades, possibly reflecting direct selection to enhance leaf thermal regulation and hydraulic efficiency in arid climates (Stojnić et al., 2022).
At the scale of a forest ecosystem, a correlation has been found between different leaf morphotypes of the same genotype and the tree's position within the canopy of F. sylvatica. Leaves above (heliophilic) and within (sciaphilic) the canopy have been analyzed. The angle between the primary vein and the first secondary vein was smaller in heliophyllous leaves compared to sciaphyllous leaves, optimizing light capture (Adamidis et al., 2021; Denk, 1999a). A similar trend is observed in isolated trees or at forest edges, where leaves have a narrower base angle. However, some populations consistently exhibit leaves with acute base angles regardless of their position in the forest (Denk, 1999a). Thus, the morphological plasticity of F. sylvatica is highly significant (Gratani, 2014).
Despite extensive studies, many morphological characteristics have not been thoroughly examined, complicating the delineation of species boundaries. For modern taxa, genetics can complement morphological studies, which is not possible for fossils. In the samples studied here, specimens belonging to the same species (F. sylvatica) show considerable variation. This variation is evident along the two factorial axes (Figure 2). However, among specimens of the same species, there are no variations in latitude, climate, or geological era. Leaves from F. sylvatica in the Fontainebleau forest have wider base angles than those from a solitary tree on the Beaulieu campus. Additionally, differences in altitude between these two groups could impact the base shape of the samples, affecting the base angle (Denk, 1999a, 1999b). The variability in F. sylvatica leaves could therefore be explained by the tree's position within a forest or by altitude.
Figure 2: Principal component analysis (PCA) showing specimens distributed according to the two factorial axes PC1 and PC2, with the four vectors influencing them the most (red arrows): the base angle (BA), the angle of the second veins (VAII), the density of the third veins (VDIII), and the angle of the third veins (VAIII). Specimens of the same species are outlined by a colored ellipse: F. grandifolia (purple, FG), F. kraeuselii (blue, FK), F. menzelii (pink, FM), F. sylvatica (yellow [dashed, 2023 samples; dotted, 1917 samples], FS), jurassian samples (green, H), and Q. lancifolia (brown, QL).
The group comprising F. grandifolia, F. kraeuselii, and F. menzelii, is relatively homogeneous, with common intraspecific variability. However, specimens are dated from Zanclean to present times, located on different continents, and associated with distinct climates (Table 1), making it challenging to determine the common factor explaining these morphological variations. Furthermore, a single specimen of F. kraeuselii is grouped with samples of F. sylvatica collected in 2023 (Figure 3), which are associated with vastly different dates and climates (Table 1). This F. kraeuselii specimen may have been isolated or at the forest edge, explaining this similarity, or may have been at a similar altitude. Similarly, two specimens of F. kraeuselii and one specimen of F. menzelii morphologically resemble the group of F. sylvatica collected in 1917 (Figure 3), despite being associated with very different environments (Table 1). Another parameter such as altitude or substrate may explain the similarity between these leaves, but since this information is unavailable, it is difficult to make these hypotheses.
Figure 3: Hierarchical cluster analysis (HCA) showing the morphological distances between the specimens. The highlights include: a group primarily containing F. sylvatica specimens collected in 1917 (yellow); a group mainly comprising F. sylvatica samples collected in 2023 and most of the jurassian samples (green); and a group containing nearly all specimens of F. grandifolia, F. kraeuselii, and F. menzelii, along with jurassian specimens (blue). The yellow and green arrows indicate jurassian samples and F. sylvatica specimens, respectively.
4.2. Concept of species
Several methodologies have been proposed to define the assignment of a fossil to a species. Some studies advocate for the differentiation of numerous species based on specific criteria. For example, four species of Fagus, including F. menzelii and F. kraeuselii, could be distinguished based on epidermis, leaf morphology, and fruit morphology during the Cenozoic in Central Europe (Kvaček and Walther, 1991). These variations indicate either species evolution over time (Kvaček and Walther, 1991) or intraspecific polymorphism (Kvaček and Walther, 1989). The second approach suggests reducing the number of established species due to the wide morphological variability observed among current species. In the genus Fagus, modern species have either extensive or restricted distributions. By parsimony, it is assumed that the fossil specimens found were not part of isolated groups but rather had distributions as vast as modern species, and thus also exhibited variability in morphological characteristics. Fossils can be categorized based on morphological criteria, considering the intraspecific variability of modern species (Denk, 2004).
In contrast to the differentiation proposed by Kvaček and Walther (1991), fossils of F. kraeuselii and F. menzelii were initially considered part of the same species and grouped under the name F. haidingeri (Von Kováts, 1856). This name referred to a recurring morphotype throughout the Cenozoic in Central Europe during the Miocene and Pliocene (Denk, 2004). These fossils are suggested to have affinities with most modern species of the Fagus subgenus found in Eurasia, including specimens identified as belonging to F. sylvatica, but excluding the North American species F. grandifolia (Denk, 2004; Kvaček and Walther, 1991).
Another comparison of morphological characteristics concludes that F. sylvatica descends from F. kraeuselii, which itself is a descendant of F. menzelii, while F. grandifolia belongs to another distinct branch (Kvaček and Walther, 1991). This hypothesis is based on the variation in the number of secondary veins, which varies greatly among species (Denk, 2004). Using other criteria, a different pattern emerges. According to Figure 2, characteristics related to secondary veins (angles) are crucial in establishing correspondences between samples. Unfortunately, the number of secondary veins could not be calculated because none of the Bletterans samples were completely intact. However, this method can sometimes directly contradict nomenclature. Linnaeus' (1753) initial description of F. sylvatica stated that its leaves lack teeth. However, in an effort to group specimens under a single name, some samples with toothed leaves were classified as F. sylvatica. The species F. moesica (Czeczottowa, 1935) was thus presented as a synonym of F. sylvatica (Denk, 1999b) despite being predominantly toothed.
The results from Figures 2 and 3 differ significantly from the presented models. A strong affinity is observed between F. grandifolia, F. kraeuselii, and F. menzelii, while F. sylvatica specimens appear isolated. A similar dichotomy is found with certain European Cenozoic specimens designated as related to the "F. sylvatica group" or the "F. grandifolia group." These are defined based on leaf length/width ratios, with oblong leaves corresponding to the first group and more elongated leaves to the second one (Tralau, 1962). Variation in leaf elongation is commonly observed in F. sylvatica specimens, and it is suggested that more elongated forms are advantageous in warm environments with high water loss (Stojnić et al., 2022). However, leaf length/width ratio does not explain the variation observed in Figures 2 and 3. Nevertheless, it would be appropriate to associate groups formed under these designations. Indeed, the dendrogram (Figure 3) clearly identifies several "groups" associated with different morphotypes. Denk (1999a) specifies that this term "group" refers to a grouping of individuals within a taxon that share morphological similarities. In the genus Fagus, morphotypes have been defined primarily at the species level (Denk, 1999a, 1999b). Similarly, F. sylvatica morphotypes were established to determine existing intraspecific variability and to define characteristics of the orientalis and sylvatica subspecies. These morphotypes are distributed according to parameters such as altitude and climate (Denk, 1999a). It is noted that some F. sylvatica morphotypes are morphologically very similar to F. grandifolia. These studies aim to define intraspecific variation based on variability observed between these subspecies.
Regarding the study of fossil material and based on the results obtained, it is possible to define morphotypes at the scale of the Fagus genus, which would denote environmental variations rather than genetic groups. The three distinct groups identified would therefore be three separate morphotypes specific to three different environments. The concept of ecotype could even be applied. Leaves of F. sylvatica thus differentiate into two distinct morphotypes, while the North American species F. grandifolia and the European species F. kraeuselii and F. menzelii would form a third morphotype. As mentioned, it is challenging to define what these morphotypes correspond to with current information. It is possible that the morphotype containing F. sylvatica specimens from 2023 is rather isolated or at the forest edge, while those of F. sylvatica from 1917 are more within closed forest environments. Parameters such as altitude or soil type that were not included, may affect the definition of these morphotypes.
4.3. Samples from Bletterans
The samples studied here are morphologically close to the three groups described previously. However, they do not show intraspecific variation along the same axis as these groups (Figure 2), primarily being distributed along PC2. Their variation is thus primarily affected by the angle of secondary veins, but also by the angle at the base, unlike other samples which are more affected by the latter. PC1 is largely influenced by the angle at the leaf base, which could not be measured in many specimens (18% of samples). With this information, it is possible that the jurassian samples would have shown a similar trend to others in the PCA.
The Bletterans samples fall into three different groups: the group of F. sylvatica collected in 2023, the group of F. grandifolia, F. kraeuselii, and F. menzelii, and, by one specimen, the group of F. sylvatica collected in 1917 (Figure 3). It is strictly impossible to assign them to the species name of F. sylvatica. As mentioned earlier, the leaves of F. sylvatica are defined as lacking denticles (Linnaeus, 1753), yet they are clearly present on the jurassian samples (Figure 1). Fagus samples found in Europe from the late Miocene were identified as F. haidingeri (Denk, 2004) (including F. kraeuselii and F. menzelii grouped here) (Figure 3). However, the Bletterans samples belong to all three groups and are not exclusively close to F. haidingeri. One unique sample is close to the morphotype of F. sylvatica collected in 1917 (Figure 3). The variation in jurassian leaves is mainly governed by the angle between the primary vein and the secondary veins: variation in light exposure could explain this difference (Adamidis et al., 2021; Denk, 1999a). Thus, some samples may have been more exposed to light than others, possibly in a more open environment like a clearing, while others were more closed under the canopy. Several hypotheses can be suggested to explain the morphological diversity of the Bletterans fossils. The hypothesis of altitude variation seems inappropriate, as the nodules from which the samples originate were extracted from a plateau in the Seille valley with little variation in altitude. Finally, other environmental characteristics not considered here, might have affected the leaf morphology of the samples. Many criteria were not visible in a large number of samples, with some varying widely depending on the environment and climate. This lack of data necessarily skews the results. Associating variation in tertiary vein characteristics with temperature and rainfall fluctuations (Uhl, 2014) is not feasible given the limited data available on the samples.
Conclusion
While some authors place F. kraeuselii and F. menzelii within the species F. haidingeri, classifying F. sylvatica and F. grandifolia separately, assigning jurassian fossils to a species is complicated. Indeed, three morphotypes have been identified: two F. sylvatica morphotypes differing by environment and season, and the morphotype grouping the majority of F. grandifolia, F. kraeuselii, and F. menzelii samples. It is challenging to define a species when it shares morphotypes with other species. In this study, two F. kraeuselii specimens are within the F. sylvatica morphotype collected in 1917. This contradicts Denk's proposition that excludes F. sylvatica from the species F. haidingeri, which includes F. kraeuselii. The Bletterans leaves are distributed across the three described morphotypes, predominantly in the F. sylvatica morphotype collected in 2023 and the morphotype grouping the majority of leaves from the species F. grandifolia, F. kraeuselii, and F. menzelii. This distribution does not allow the assignment of a defined species to these samples but supports the presence of leaf morphotypes adapted to different environments. The study of several species present in the flora of the Bletterans plateau, along with an analysis of complete specimens, could provide more precise indications of the environment during the Piacenzian in this locality, thereby better understanding the intraspecific variations of the Bletterans samples.
The concept of species has always been very difficult to establish in palaeobotany. Historically, palaeobotanists have worked against the rules of naturalism and taxonomy, naming new taxa based on fragmentary remains without considering phenotypic plasticity. For purely ego-related reasons, the multiplicity of synonymous genus and species names has made the study and understanding of fossil plant species complicated, if not impossible. Naming a new fossil species resembles more a childish game than a scientific one, whereas acknowledging polymorphism would greatly simplify this science and make it more consistent with current observations of living organisms.

![Figure 2: Principal component analysis (PCA) showing specimens distributed according to the two factorial axes PC1 and PC2, with the four vectors influencing them the most (red arrows): the base angle (BA), the angle of the second veins (VAII), the density of the third veins (VDIII), and the angle of the third veins (VAIII). Specimens of the same species are outlined by a colored ellipse: F. grandifolia (purple, FG), F. kraeuselii (blue, FK), F. menzelii (pink, FM), F. sylvatica (yellow [dashed, 2023 samples; dotted, 1917 samples], FS), jurassian samples (green, H), and Q. lancifolia (brown, QL).](docannexe/image/1235/img-2-small600.jpg)