in Silico Pharmacology – Molecular Modeling
Last modifications: 02/08/2019 by Florent
Staff
- Prof. Patrick Trouillas
- Dr. Florent Di Meo
- Dr. Gabin Fabre
- Dr. Petra Cechova
- Michaela Melikova
- Marving Martin
- Mehdi Benmameri
- Agotá Tóth
Funding/Projects
- IMOTEP (ANR – National)
- MemCross (NA – Regional)
- VICTOR (NA – regional)
Partnership
There is a need for deeper understanding and finer quantification of drug blood concentration – effect (PK/PD) relationships, for which interactions between cell membrane and xenobiotics play a crucial role. Drug-membrane crossing is directly involved in local PK, i.e., drug concentration at the target sites, whether linked with drug therapeutic or adverse effects.
The clinical assessment of local PK of ISD that directly affects drug response is still challenging (e.g., evaluation of intracellular concentration of tacrolimus in lymphocytes or kidney cells, respectively responsible for drug action or drug adverse effect).
Drug Insertion and Passive Permeation
Drug-membrane interactions are even more important when the biological action lies inside the membrane. Despite their great importance in terms of pharmacokinetics (PK) and pharmacodynamics (PD), drug-membrane interactions are, in many cases, poorly understood.
Our different projects have dealt with the incorporation of various compounds in lipid bilayers by means of MD simulations, allowing predicting the penetration depth and orientation of these compounds, with respect to membrane composition.
Although the biological action or toxicity of xenobiotics are often well documented, mainly from biological assays nowadays joint to a physical-chemical understanding, the knowledge about membrane crossing is still fragmented. This relies from the difficulty to capture this highly dynamic process.
Besides, it is well-admitted that drug passive permeation can play a key role in PK/PD. Based on a long-term and solid expertise acquired over the past decade of the different partners, we aim at developing a robust predictive expert system to comprehensively evaluate the capacity of a given xenobiotic to partition in or permeate through lipid bilayer membranes passively.
Protein-Mediated Transport
Particular attention should be paid to human transporters that are major actors of drug membrane crossing events. For instance, the International Transporter Consortium (ITC) described a series of human transporters as being of « emerging clinical importance », which was approved by both the European Medicines Agency (EMA) and the Food and Drug Administration (FDA). Among these transporters, the organic anion transporting polypeptides OATP1B1 and OATP1B3, the organic anion transporters OAT1 and OAT3.
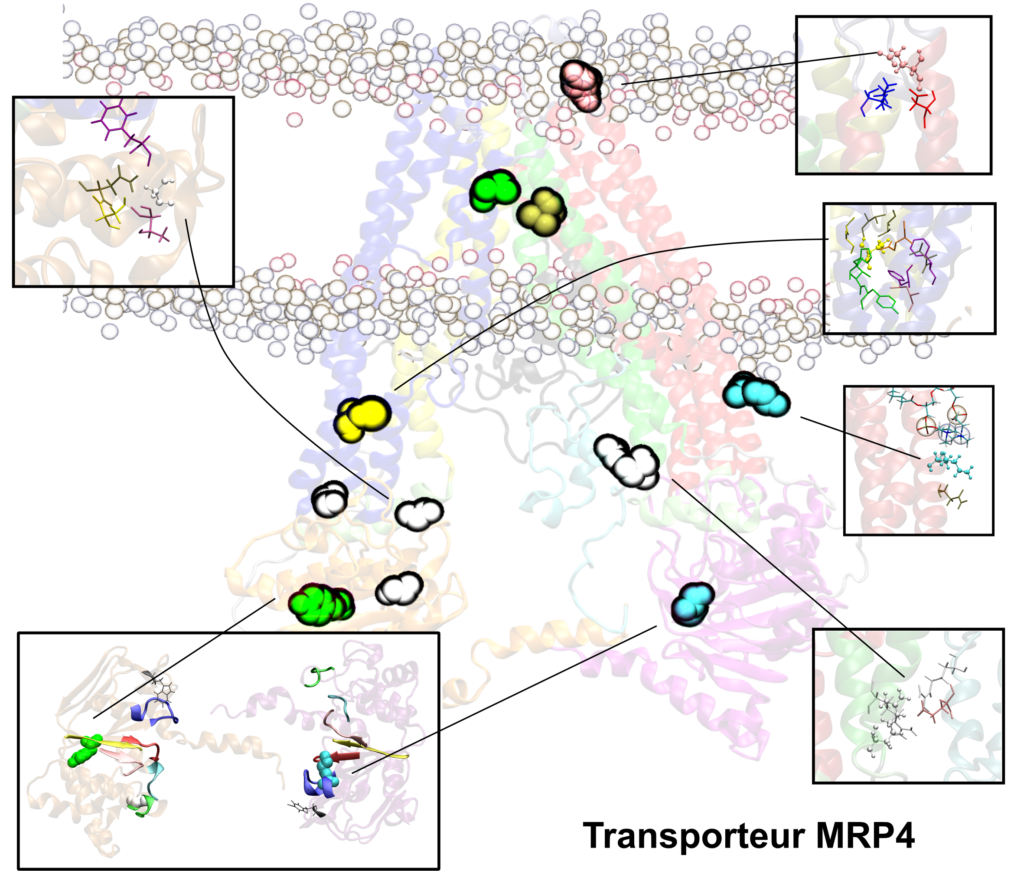
We are also studying the multidrug resistance-associated proteins MRP2 and MRP4 which are known or expected to interact with the most commonly used ISD (e.g., cyclosporine, tacrolimus, mycophenolic acid) but also with other drugs widely prescribed to immune depressed organ transplant patients (e.g., nucleic acid-like antivirals, cephalosporins).
All our projects are in strong collaboration with Prof. Michal Otyepka‘s group, at RCPTM – Palacky University Olomouc, Czech Republic and Prof. Claire Rossi, at UTC, France.
The development of atomic-based in silico pharmacological models of membrane transporters may represent a step forward in the understanding of drug PK and PD by adding a new size- and timescale to PK/PD and PGx tools. The main goal is to provide a comprehensive overview of drug membrane transporter to support experimental and clinical data as a complement to already existing predictive tools
These in silico transporter models will be built to picture the membrane with a specific composition and will be used to simulate difficult-to-measure, poorly documented or new conditions, mainly:
- Dynamics of the transporter proteins
- The influence of different polymorphisms (e.g., single nucleotide polymorphisms – SNP) or rare mutations
- Investigation of DDIs occurring in contact with the membrane transporter
Clinical Relevance
We strongly believe in this (predictive) in silico pharmacological approach for a near-future clinical usage. Many sources of inter-patient variability in drug responses, only a fraction of which have been identified, lie at the molecular level. Modeling the mechanisms of drug uptake in, and efflux from, target cells and tissues may help identify such sources and infer their global influence.
The in silico membrane transporter models will first be validated on ganciclovir, mycophenolic acid and calcineurin inhibitors that are relevant prototypes for which in vitro and in vivo experimental data are available in our unit. The atomic membrane transporter models will further be used to predict membrane crossing by many other drugs of interest, and drug-drug interactions at the transport protein level.