|
The Flory-Huggins isotherm
|
The Flory-Huggins adsorption isotherm is defined by the following equation:
where b is the equilibrium binding constant, c the concentration
of the adsorbate in solution, θ the fraction of adsorbent sites
occupied by the adsorbate and n a positive exponent.
The equation can be linearized as follows:
|
ln |
θ
c
|
= lnb + n ln(1 − θ) |
|
Unfortunately, some authors have replaced, in the previous equation,
the variable c
by a constant c0, resulting in an equation in the single
variable θ. This mistake has considerably hampered the use of
the isotherm!

Figure 1: Our investigation reveals that the Flory-Huggins adsorption
isotherm was destroyed beyond recognition by a farcical linearization
method. It is not a pretty picture. We are on a mission to resurrect
the original isotherm. Let's dig in. (Graphic credit: Aan Chu)
Fitting the Flory-Huggins isotherm
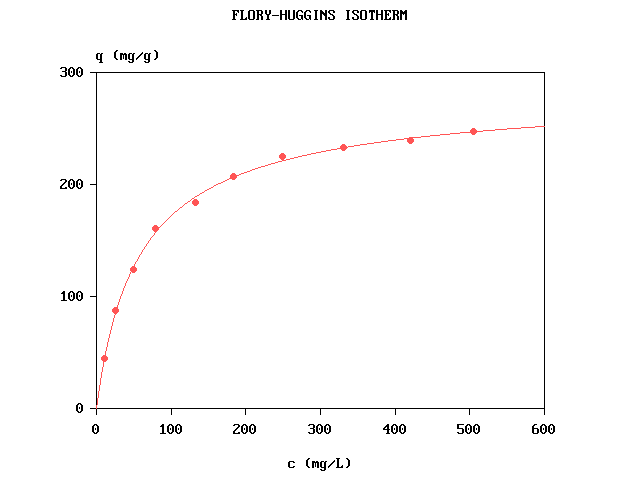
In practice, θ is computed as θ = q / qm where q is the adsorbed
quantity and qm its maximal value (adsorption capacity). The experimental
curve is therefore defined by q = f(c).
The computer program Flory_Huggins.bas written in FreeBASIC fits the curve by nonlinear
regression, using simulated annealing followed by Marquardt's method. The fitted parameters are
qm, b and n. No linearization is used.
Reference
K. H. Chu, M. A. Hashim, H. Bashiri, J. Debord, M. Harel, J. C. Bollinger.
The Flory-Huggins Isotherm and Water Contaminant Adsorption: Debunking Some
Modeling Fallacies. Industrial & Engineering Chemistry Research,
2023, 62, 1121−1131
https://pubs.acs.org/doi/10.1021/acs.iecr.2c03799


